Ion Microprobe Analysis of Oxygen Isotope Ratios in Granulite Facies Magnetities: Diffusive Exchange as a Guide to Cooling History
Valley, John W. and Graham, Colin, M. (1991) Ion microprobe analysis of oxygen isotope ratios in granulite facies magnetites: diffusive exchange as a guide to cooling history. Contributions to Mineralogy and Petrology, v. 109, p. 38-52
Abstract
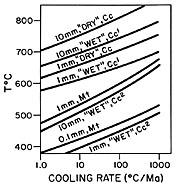
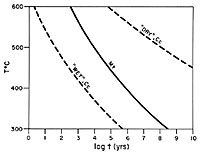
Ion microprobe analysis of magnetites from the Adirondack Mountains, NY, yields oxygen isotope ratios with spatial resolution of 2-8 m m and precision in the range of 1‰ (1 sigma). These analyses represent 11 orders of magnitude reduction in sample size compared to conventional analyses on this material and they are the first report of routinely reproducible precision in the 1 per mil range for analysis of d 18O at this scale. High precision micro-analysis of this sort will permit wide-ranging new applications in stable isotope geochemistry. The analyzed magnetites form nearly spherical grains in a calcite matrix with diopside and monticellite. Textures are characteristic of granulite facies marbles and show no evidence for retrograde recrystallization of magnetite. Magnetites are near to Fe3O4 in composition, and optically and chemically homogeneous. A combination of ion probe plus conventional BrF5 analysis shows that individual grains are homogeneous with d 18O = 8.9 ± 1‰ SMOW from the core to near the rim of 0.1-1.2 mm diameter grains. Depth profiling into crystal growth faces of magnetites shows that rims are 9‰ depleted in d 18O. These low d 18O values increase in smooth gradients across the outer 10 m m of magnetite rims in contact with calcite. These are the sharpest intracrystalline gradients measured to date in geological materials. This discovery is confirmed by bulk analysis of 150-350 m m diameter magnetites which average 1.2‰ lower in d 18O than coarse magnetites due to low d 18O rims. Conventional anlaysis of coexisting calcite yields d 18O = 18.19, suggesting that bulk D 18O (Cc-Mt)=9.3‰ and yielding an apparent equilibration "temperature" of 525° C, over 200° C below the temperature of regional metamorphism. Consideration of experimental diffusion data and grain size distribution for magnetite and calcite suggests two contrasting cooling histories. The data for oxygen in calcite under hydrothermal conditions at high P(H2O) indicates that diffusion is faster than in magnetite and modelling of the low d 18O rims on magnetite would suggest that the Adirondacks experienced slow cooling after Grenville metamorphism, followed by a brief period of rapid cooling, possibly related to uplift. Conversely, the data for calcite at low P(H2O) show slower oxygen diffusion than in magnetite. Modelling based on these data is consistent with geochronology that shows slow cooling through the blocking temperature of both minerals, suggesting that the low d 18O rims form by exchange with late, low temperature fluids similar to those that infiltrated the rock to serpentinize monticellite and which infiltrated adjacent anorthosite to form late calcite veinlets. In either case, the ion microprobe results indicate that two distinct events are recorded in the post-metamorphic exchange history of these magnetites. Recognition of these events is only possible through microanalysis and has important implications for geothermometry.
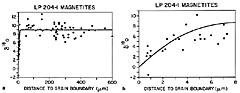
| Figure 6a, b. Values of d 18O in magnetite in a composite of data from 11 crystals measured on six days plotted against distance from the magnetite grain boundary (Table 3). Results from depth profiles (<8 m m) are consistent with spot analyses (>50 m m) indicating that these 0.1-1.2 mm diameter grains are homogeneous from core to within 10 m m of the rim, and that the outer 10 m m rim is depleted in d 18O with a gradient of 9‰/10 m m. a shows all data (1-8, 50-600 m m); b is an enlargement of the depth profile data showing an inverse error function curve, fit to the data and characteristic of volume diffusion |