SIMS Analysis of Oxygen Isotopes: Matrix Effects in Complex Minerals and Glasses
Eiler, John M., Graham, Colin, Valley, John W. (1997) SIMS analysis of oxygen isotopes: matrix effects in complex minerals and glasses. Chemical Geology, v. 138, pp. 221-244. View full-text pdf
Abstract
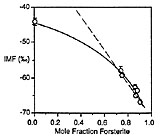
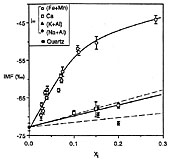
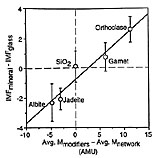
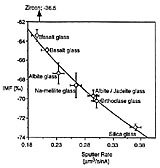
A large instrumental mass fractionation (IMF) occurs during the measurement of oxygen isotope ratios by secondary ion mass spectrometry and the magnitude of this fractionation can be dependent upon sample chemistry, resulting in so-called ‘matrix effects’. We have made 373 measurements of the 18O-/16O- ratio (±0.5-1.1‰ internal precision, 1 s.d.) in 40 silicate and phosphate minerals and glasses for the purpose of characterizing this matrix effect. The magnitude of IMF decreases with increasing atomic mass of the sample, although this relationship is too poorly defined to be the basis of a precise empirical correction scheme. IMF is well correlated with simple measures of chemistry among related minerals and glasses (e.g. forsterite content in olivine; Na/(Na + K) in feldspar glass), and with the atomic abundance of certain elements among several mineral groups (e.g. the sum: (Fe + Mn) in garnets, olivines and pyroxenes). Significant differences in IMF between minerals and glasses of the same chemical composition correlate with the mass of network modifying cations. Instrumental mass fractionation correlates strongly with sputter ratio for albite and seven silicate glasses.
Two mechanisms for producing matrix effects are proposed: (1) differences in the efficiency with which kinetic energy is transferred to the secondary oxygen atoms from the atoms in the near-surface region of the sample, and (2) simple variations in the fraction of sputtered oxygen atoms that are ionized, such that the observed instrumental fractionation diminishes as that fraction approaches 1. The correlation between sputter rate and instrumental mass fractionation in silicate glass and albite is well predicted by model (1). Our data suggest that a large range of silicate materials can be standardized using: (1) interpolation among chemically similar standards; (2) correlation of IMF with Fe + Mn content in materials rich in those elements; (3) model (1), particularly for materials that are compositionally related to standards.
One or more of these methods appear to be applicable to most silicate materials of interest and should permit accurate standardization of ion probe analyses without the stringent requirements that standards and unknowns be compositionally identical or compositionally similar members of the same solid solution series.